AI checks pharmaceutical "manufacturing and sales approval documents"! Macnica develops text matching AI system for the pharmaceutical industry - resolving the lack of inspection resources and human error -
Macnica (Headquarters: Yokohama, Kanagawa Prefecture; Representative Director and President: Kazumasa Hara; hereinafter Macnica) announced today that it is working on developing a checking system that can automate the checking of consistency between manufacturing and sales approval documents for pharmaceutical companies and the actual manufacturing and testing methods, thereby reducing the amount of work required and increasing reliability. In preparation for service launch, the company is now looking for test users.
Background
Various initiatives are being undertaken across the industry, including simultaneous inspections based on an administrative notice in 2016 *1 and voluntary inspections based on the Japan Generic Pharmaceuticals Association's checklist in 2021, with the aim of reducing discrepancies and inconsistencies between manufacturing and marketing approvals and actual manufacturing conditions.
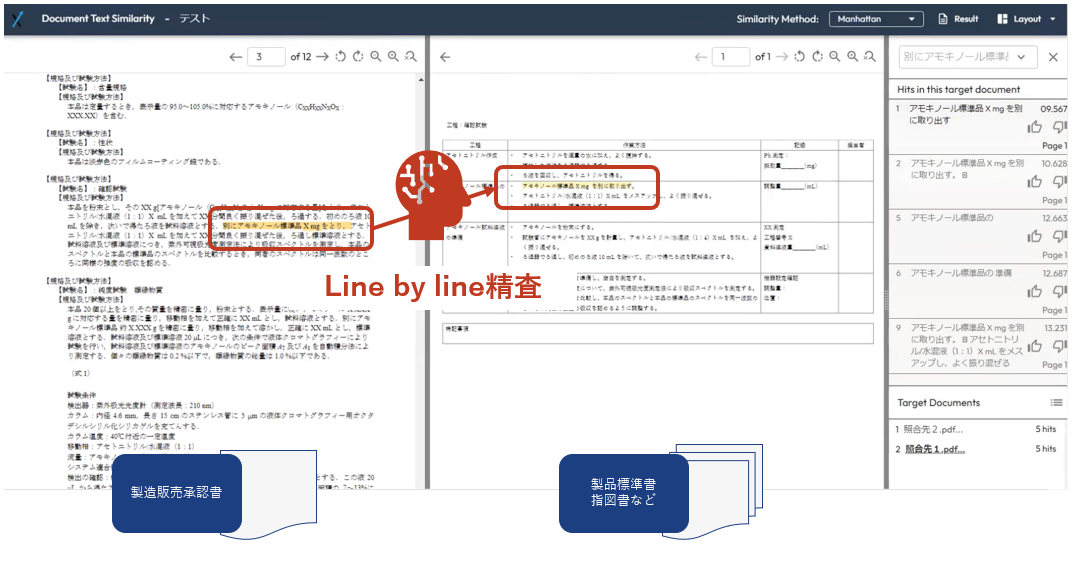
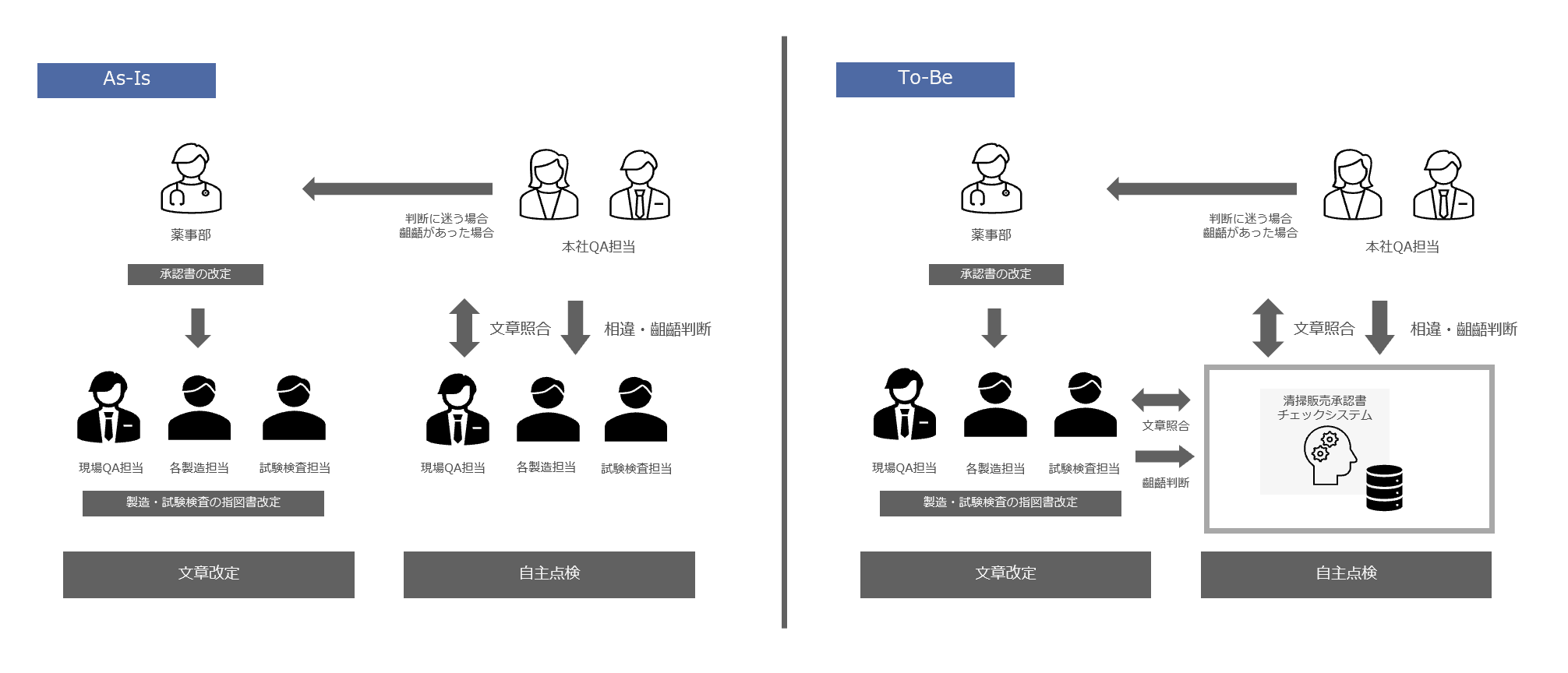
In the voluntary inspection, the manufacturing and sales approval checks were conducted by the manufacturing and testing inspection staff of each process in rotation, who conducted face-to-face checks. The manufacturing and sales approvals, product standards, instructions, and other documents were printed out on paper, and the manufacturing and sales approvals were divided into sections, which were then checked line by line while being compared with the contents of the vast number of product standards and instructions.
This type of self-inspection method can improve quality if sufficient time and manpower are secured, but in reality, there are always challenges such as lack of resources and human error.
■ Solution Overview
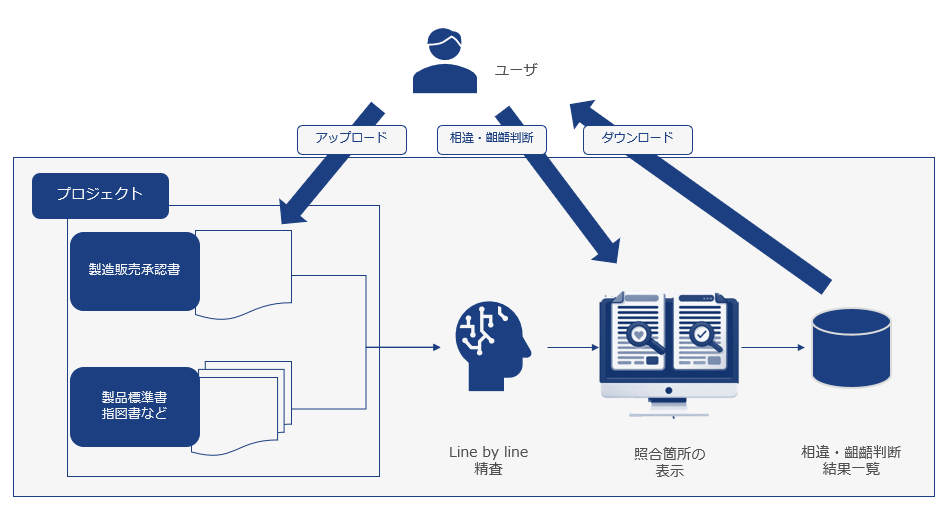
This system uses AI to automatically extract and verify passages from manufacturing and sales approval documents from a huge amount of text, such as product standards and instructions. It also clearly indicates where the relevant passages are written, helping QA personnel to identify discrepancies and inconsistencies.
Feature 1: We implement an AI model with high text matching accuracy that reduces work time based on manufacturing and sales approvals, product standards, instructions, etc. from development partner companies.
Feature 2: Based on feedback from three pilot customers and multiple monitors over a period of more than a year, we provide a user-friendly UX that is tailored to your business needs.
<Screen image (alpha version)>
<Function flow diagram>
■ Benefits of implementation
The following benefits are expected from the introduction of this system in order to comply with GMP *3 and GQP *4 ministerial ordinances.
Effect of introduction 1: Practical self-inspection:
To resolve the issue of resource shortages caused by time constraints, we support you by reducing the time required for inspections through the digitalization of self-inspections, thereby improving the efficiency of inspection work.
Benefits of Implementation 2: More reliable compliance with laws and regulations:
In order to reduce human error caused by manual work, we will automate some tasks using AI, automating line-by-line inspections and helping to improve the quality of self-inspections.
■Recruiting test users
Macnica is currently recruiting test users to further improve its quality.
To apply: https://go.macnica.co.jp/CAX-Inquiry-Form.html
■ Macnica 's generative AI solution
Macnica sources cutting-edge technology from around the world, with semiconductors and networks at its core, and implements it to solve the problems of a wide range of customers. With regard to AI, the company has had an in-house research team from an early stage and is promoting its use in new businesses, such as data analysis. By incorporating AI, data that could not be fully utilized until now can be made to have great value. It can also be a catalyst for business transformation, such as overwhelming improvements in operational efficiency. Macnica will lead social transformation by answering the challenges of partner companies and accompanying them.
*1: Notification from the Director of the Inspection and Licensing Division, Pharmaceutical and Food Safety Bureau, Ministry of Health, Labour and Welfare, addressed to the Directors of the Health Departments of each prefecture, PSEHB-Evaluation and Licensing Division No. 0119-1, regarding the implementation of inspections for consistency between pharmaceutical manufacturing and sales approvals and actual manufacturing practices
https://www.mhlw.go.jp/web/t_doc?dataId=00tc1544&dataType=1&pageNo=1
*2: Japan Generic Medicines Association: Measures to ensure the reliability of generic drugs
https://www.jga.gr.jp/assets/uploads/2021/920381a441fdd5a32dcfcb081582fd28b2fc4e3f.pdf
*3: Good Manufacturing Practice, a standard for pharmaceutical manufacturing and quality control
*4: Good Quality Practice, a standard that stipulates methods for quality control of pharmaceuticals, quasi-drugs, cosmetics, regenerative medicine, and other products.
[Click here for product inquiries]
Pharmaceutical GQP Manager, AI Solutions Department Macnica
TEL: 045-476-2010
Email: cax-sales@macnica.co.jp
*Company names and product names mentioned in this text are trademarks or registered trademarks of Macnica and each company.
*The information published in the news release (including product price, specifications, etc.) is current as of the date of announcement. Please note that the information may be subject to change without prior notice.
About Macnica
Macnica is Service & Solution Company that handles the latest technologies in a comprehensive manner, with semiconductors and cyber security at its core. With operations in 81 locations in 23 countries/regions around the world, the company is leveraging the technical capabilities and global network it has cultivated over its 50-year history to discover, propose, and implement cutting-edge technologies such as AI, IoT, and autonomous driving.
About Macnica: www.macnica.co.jp
Inquiries from the press regarding this matter
Macnica, Inc. https://www.macnica.co.jp
Public Relations Office, Miyahara, Isozaki. E-mail: macpr@macnica.co.jp
Macnica,Inc. 1st Building, 1-6-3 Shin-Yokohama, Kohoku-ku, Yokohama 222-8561