
近年、様々な分野でデジタルトランスフォーメーション(DX)の必要性が叫ばれておりますが、それは医療分野でも同様です。ITを使った業務効率化はもちろんのこと、AIやデータを用いて既存業務を変革し、顧客に新たな価値・体験を提供する「攻めのデジタル化」へのシフトが迫られています。ここでは、「データ×医療」をテーマとした3つのセッションから、日本における医療テクノロジーの現在地や、DXの進めるうえでのポイントについて迫っていきます。
AIプログラム医療機器の課題と規制
新たな医療機器を開発した際には、分類に合わせて認証や承認を受ける必要があります。認証は専門の認証機関に申請し、承認は日本ではPMDA(医薬品医療機器総合機構)で審査が行われます。医療機器は政令で種類(カテゴリ)が定められており、2014年に「プログラム医療機器」というカテゴリが作られています。
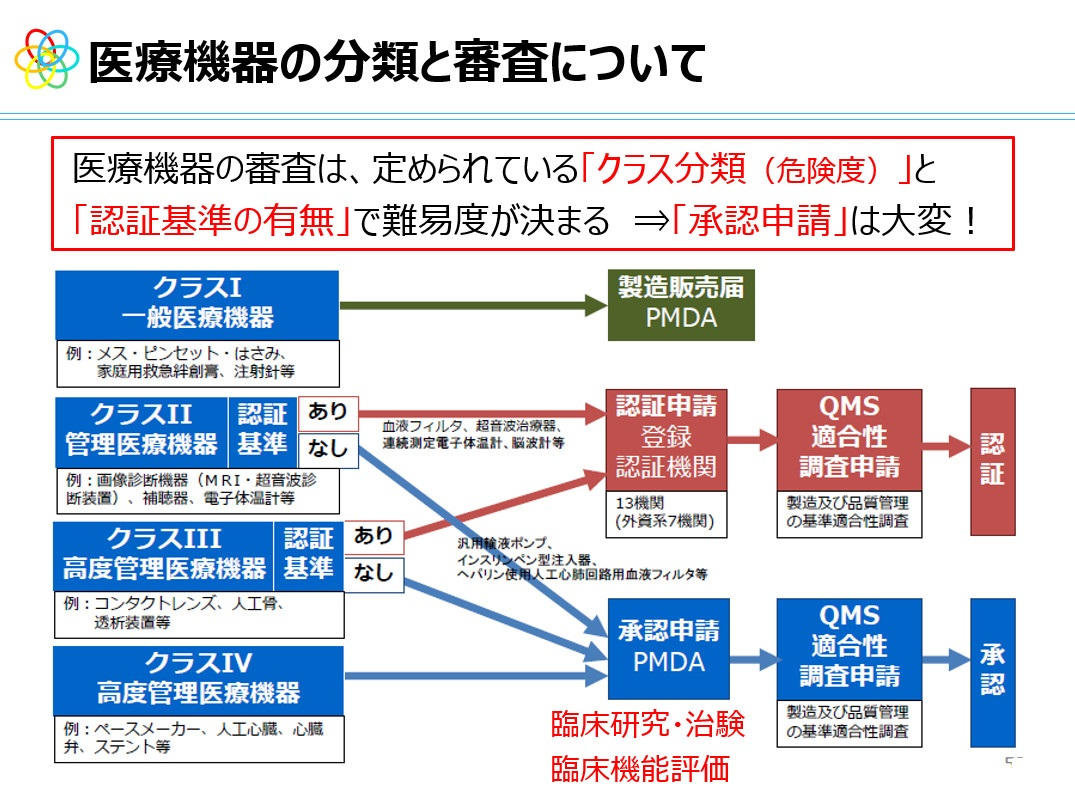
医療機器の分類と審査
プログラム医療機器には、診断用、治療用、予防用の「プログラム(医療機器プログラム:SaMD)」、および3種類の「プログラムを記録した記録媒体」の合計6種類が設定されています。AI医療機器はこのプログラム医療機器に含まれます。ただし、例えば健康データを記録できるアプリでは、重症化の予防や病気の発見を目的に開発されたものは医療機器プログラムになるが、データの管理や記録のみが目的のものは非医療(ヘルスケアアプリ)となります。
では、AIが医療現場でどのように活用されていくのでしょうか。
アイリス 取締役副社長、CSOであり医師でもある加藤浩晃氏は「まず問診では、来院前にタブレットに入力したり、Webでの問診で入力したものがAIによって、カルテに専門用語を含めた文章が自動的に出力されます。検査や診察では、AIがCT画像などに病気が疑われる箇所にマーキングをしたり、内視鏡画像で腫瘍なのかどうかを判断したりする。これにより、効率化が進んでおり、今後、高精度化や新たな検出などが可能になる」と話します。
こうした医療AIの開発は日本でも活発に行われており、その数も増えています。一方で、AI医療機器は原則として治験(臨床試験)として実施する必要があるため、GCP(省令)への対応や手順書の作成、データのモニタリングや統計解析、PMDAへの治験届など非常に手間がかかり、費用も億単位となります。
アイリスでは、2021年6月に「のど」の撮影で感染症を診断できるAI医療機器を、前向き試験として治験を実施し、承認申請を行っています。「のど」の画像は今まで医療機関で画像データとして蓄積されていないため、カメラから作成し、一から20万枚以上の画像を集めました。
「アイリスの場合と違って、すでにある医療機関の画像データや診療情報を使う場合は、AI医療機器を治験で行わなくても短期で承認申請を実現できるようになります」(加藤氏)
AI医療機器の課題と政府動向
開発における課題には、「医療機器の該当性がわかりにくい」「開発のための良質なデータを収集することが難しい」「データ整理が大変」「保険戦略が必要」「販売後のプログラムのアップデートを勝手に行えない」などがあります。また、個人情報保護法への対応も必要になります。
担当窓口が分散していて一本化されていないことも課題です。政府の方針としては、経済財政諮問会議からの「骨太方針」に「プログラム医療機器の開発実用化を促進する」と明記されています。また、デジタル時代に向けた規制の見直しとして、「プログラム医療機器の開発・導入の迅速化に資する審査体制・制度の見直し」も令和3年度に検討・結論と書かれています。
厚生労働省では、プログラム医療機器の審査の抜本改革として「DASH for SaMD」を発表しています。これは、「萌芽的シーズの早期把握と審査の考え方の公表」「相談窓口の一元化」「プログラム医療機器の特性を踏まえた審査制度」「早期実用化のための体制強化」を挙げています。
また、医療の将来像として経済産業省においては、2040年に目指すイメージとして「どこでも医療アクセス」を発表しています。
「AI医療機器(ソフトウェア医療機器)の開発には『医療現場・医療業界間』『医療機器制度』『ソフトウェア開発』の3つの視点が必要であり、それぞれに意識すべきことや課題、求められることがある。AI医療機器の開発には、適切なステークホルダーと共創する必要があります」(加藤氏)
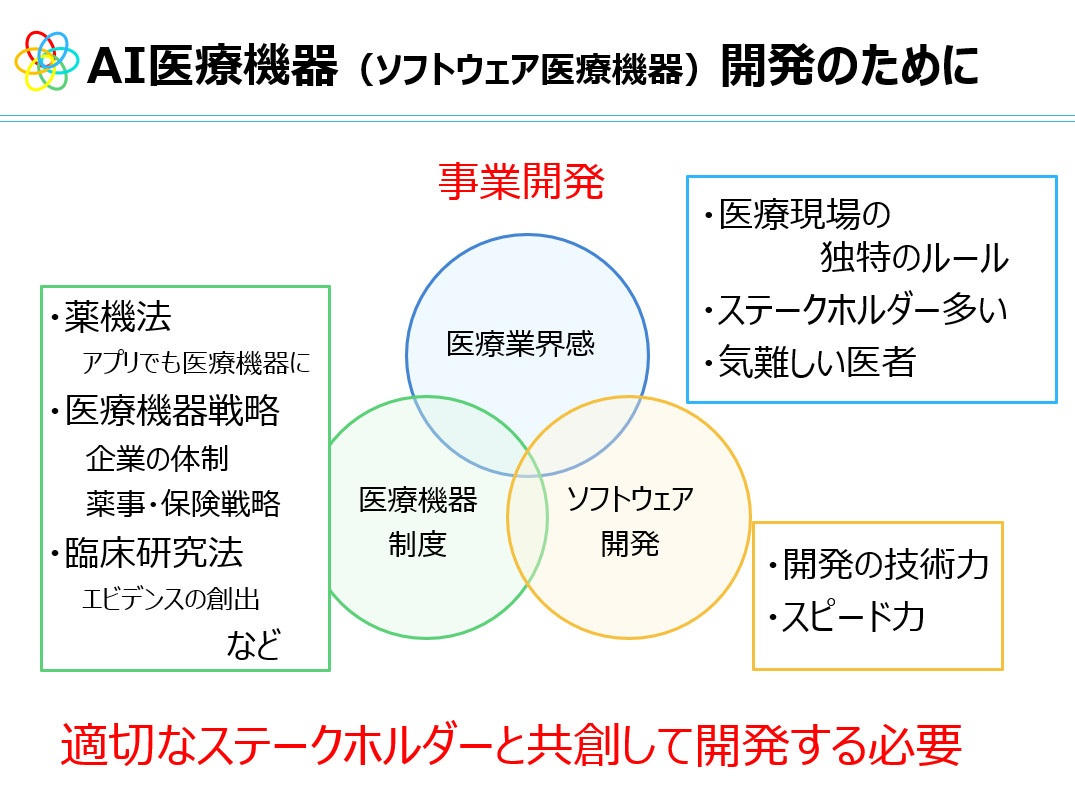
AI医療機器開発のための3つの視点
ウェアラブルデバイスは高血圧を解明する可能性
次にバイタルデータの連続モニター、その中でも血圧のモニタリングに関するテクノロジーについて見てみましょう。Arbletではコア技術として、デバイスを使った24時間連続での血圧モニタリングを開発しています。
「一般的なウェアラブルとの違いとしては、今までウェアラブルで計測されていたデータのうち、捨ててしまっていたデータを上手に活用する技術になります。多くのウェアラブルデバイスは、その裏側に緑色の光が見えます。これが光センサーであり、皮膚にLEDの光を照射し、その反射量の変化をフォトダイオードで検知しています」とArblet 代表取締役の清水滉允氏は話します。
血中内のヘモグロビンは、緑色の光を吸収する性質を持っており、この性質を利用して血液量をモニタリングします。その計測データには、手の動きや呼吸成分などさまざまな情報が含まれており、これをフィルタリングすることで、「脈波」と呼ばれる心臓の拍動が得られます。
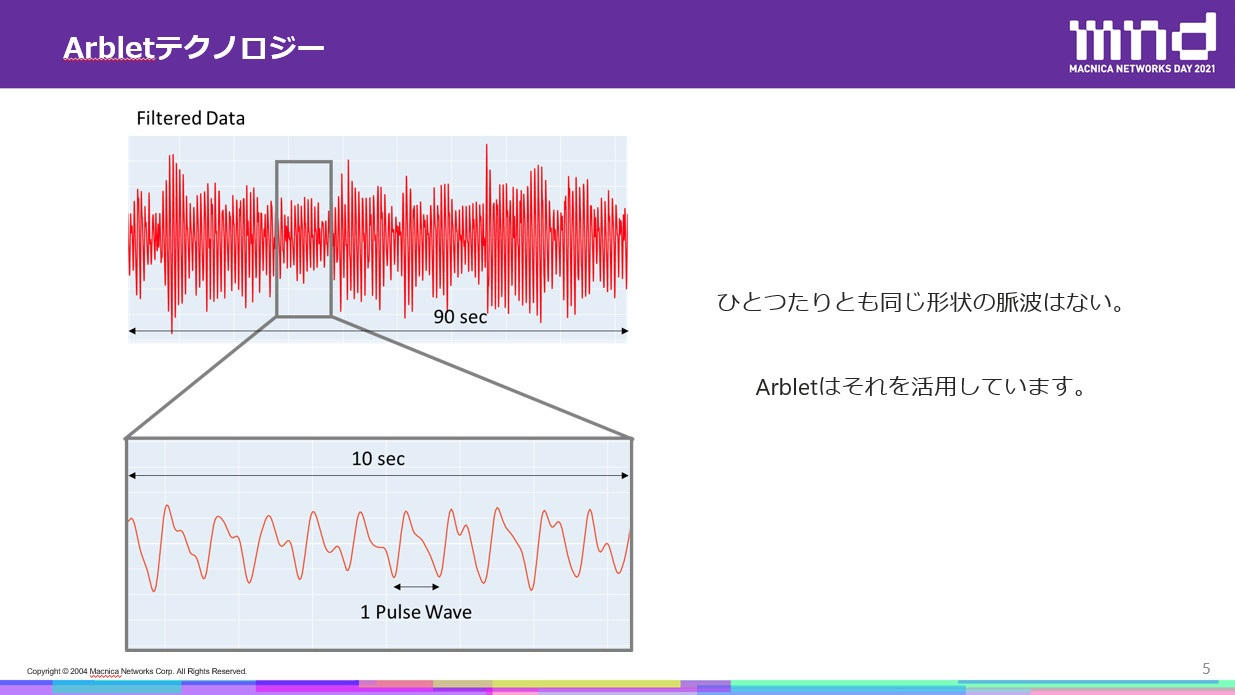
Arbletの技術により得られる「脈波」
ウェアラブルデバイスは人生のベストパートナーに
日本高血圧学会次世代高血圧学委員会の委員長であり、国際医療福祉大学大学院 医学研究科循環器内科学の教授である岸拓弥氏は、「心臓の状態を判断する材料のひとつが血圧ですが、これを一回の血圧測定で決めることは情報が足りず、数値化にも限界があります。そのためにウェアラブルデバイスが有効です。これにより、意識することなくデータを蓄積していくことができます。つまりウェアラブルデバイスは『ゆるやかな見守り』を可能する人生のベストパートナーとも言えるのです」と述べています。
ウェアラブルデバイスで測定することにより、心臓の状態をより知ることができます。これにより、本当に高血圧なのか、服用している薬は適切なのか、治療法を含めてより詳細に分かるようになります。医療分野のDXが進みウェアラブルデバイスのデータを活用できるようになれば、それを解決する可能性があるでしょう。
医療・製薬分野で「攻めのデジタル化」が加速
また医療健康分野のデータ活用と製薬企業から見たブレインテックの可能性についても言及しています。
新薬を世に出すまでには非常に長い時間と費用が必要で、しかも成功確率は実に2万5,000分の1と非常に低いという産業の特性があります。一方で、日本の基幹産業として非常に重視されており、日本製薬工業協会(製薬協)においても産業ビジョンを実現する方法のひとつとしてDXを掲げています。
製薬産業では、業務効率化を目指したデジタル化の一環として、AI創薬やバーチャル治験、RPAの導入などが進んでいる一方で、デジタルセラピューティクス(DTx、日本では「治療用アプリ」と呼ばれることも)や顧客に新たな体験価値を提供するための疾患管理アプリの提供、デジタルバイオマーカーの開発にも取り組んでいます。
Meiji Seika ファルマ 経営企画部 デジタル推進グループ長の佐々木隆之氏は「これまでは公的医療保険のなかで事業を行っていた医薬品産業ですが、医療・ヘルスケアのパラダイムシフトへの対応が進み、健康寿命の延伸を目指して予防や食、衣服、住宅などに取り組む企業も増えています。この動きは『Beyond the Pill』と呼ばれています」と説明しています。
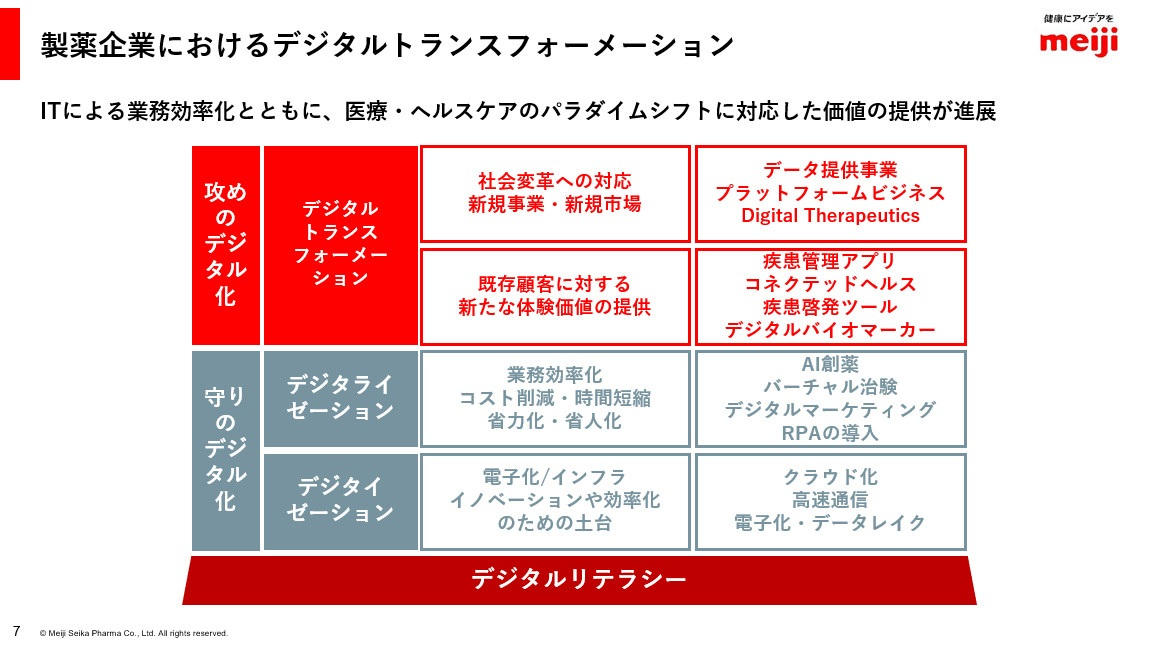
IT活用により、顧客への新たな体験・価値の提供が可能に
ブレインテックがもたらす製薬業界にもたらす可能性とは
さらに佐々木氏は「脳(Brain)」と「テクノロジー(Technology)」を組み合わせた「ブレインテック」についても言及しています。
「ブレインテックは、医療ヘルスケアの分野でも大きく期待されており、特に「活動障壁の消失」(ヒューマンオーグメンテーションやBMI)と、「脳活動の自己制御」(行動変容)の2つの分野で大きな期待が寄せられています」(佐々木氏)
では、製薬業界がブレインテックにどのように寄与できるのでしょうか。
「人体の健康に直接関わってくることですので、医療や薬事に関する法規制を理解し、遵守すること、そして質の高いエビデンスを創出することが、まずは大切です。例えば、健康のための測定・介入を行うエビデンスに基づくソフトウェアの提供や、医学的な障害や疾患を管理、治療するプログラムの提供には、医療機器プログラムとしての薬事承認が求められますし、そのためには臨床試験によって効果を証明する必要があります。」と佐々木氏は説明します。
これに対してデジタルヘルスは、「医療と健康の成果を向上させるもの」と定義をされておりますが、薬事承認の外にあります。医薬品産業から見ると、例えば服薬遵守や治療継続において、患者のモチベーションを高めるといった心理面のフォローは、ブレインテックとの親和性があります。
続々と登場するDTxソリューション
DTxの市場は今後急拡大すると見られており、2020年代後半には1兆円を大きく超える規模になると予測されています。一方、この10年でDTxも進化しており、初期のテキストコミュニケーションや数値報告ベースのDTxから、最近はゲーミフィケーションも活用しつつ「自然な形」「頑張らない形」で治療や予防にデジタルを役立てていくケースが増えています。
「日本では、厚生労働省がプログラム医療機器の実用化促進パッケージ戦略を発表し、事業を進めやすい環境の整備を進めています。これからブレインテックを考えていく企業は、こうした薬事規制の変化の把握が必要でしょう。また、ブレインテックで扱うデータのなかには機微性の高いデータもあるため、研究、診断や治療に活用することに関して、個人情報保護や倫理面から世界中でさまざまな検討がなされています。
こうした課題を解決していくには、医療のドメインを知る者と情報科学を知る者、デジタルデバイス、人間の心理や感情、こういったことを理解している者が複合的に協業していく必要があります。これにより、新しい価値の創出が可能になるのです」(佐々木氏)
▼人物キャプション
アイリス株式会社 取締役副社長CSO 医師
加藤 浩晃氏
日本高血圧学会次世代高血圧学委員会委員長
国際医療福祉大学大学院 医学研究科循環器内科学 教授
岸 拓弥氏
株式会社Arblet 代表取締役
清水 滉允氏
株式会社マクニカ 新事業本部ヘルスケア事業推進室 室長
西村 武郎氏
Meiji Seika ファルマ株式会社 経営企画部 デジタル推進グループ長
佐々木 隆之氏
株式会社マクニカ新事業本部メディカル事業推進室 室長補佐
林 靖彦氏